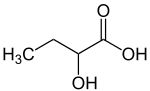
2-Hydroxybutyric acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Hydroxybutanoic acid | |
| Other names α-Hydroxybutyric acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.009.079 |
| KEGG |
|
| MeSH | 2-hydroxybutyric+acid |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C4H8O3 |
| Molar mass | 104.105 g·mol−1 |
| Related compounds | |
Other anions | hydroxybutyrate |
Related carboxylic acids | propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid hydroxypentanoic acid |
Related compounds | erythrose threose 1,2-butanediol 1,3-butanediol 2,3-butanediol 1,4-butanediol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
2-Hydroxybutyric acid, is a hydroxybutyric acid with the hydroxyl group on the carbon adjacent to the carboxyl. It is a chiral compound having two enantiomers, D-2-hydroxybutyric acid and L-2-hydroxybutyric acid. Its conjugate base is known as alpha-hydroxybutyrate and α-hydroxybutyrate.
-
 d-2-hydroxybutyric acid
d-2-hydroxybutyric acid -
 l-2-hydroxybutyric acid
l-2-hydroxybutyric acid
2-Hydroxybutyrate, the conjugate base of 2-hydroxybutyric acid, is produced in mammalian tissues (principally hepatic) that catabolize L-threonine or synthesize glutathione. Oxidative stress or detoxification demands can dramatically increase the rate of hepatic glutathione synthesis. Under such metabolic stress conditions, supplies of L-cysteine for glutathione synthesis become limiting, so homocysteine is diverted from the transmethylation pathway forming methionine into the transsulfuration pathway forming cystathionine. 2-Hydroxybutyrate is released as a byproduct when cystathionine is cleaved to cysteine that is incorporated into glutathione. Chronic shifts in the rate of glutathione synthesis may be reflected by urinary excretion of 2-hydroxybutyrate.
α-hydroxybutyrate may be useful as an early indicator of insulin resistance in non-diabetic subjects.[1] Moreover, elevated serum α-hydroxybutyrate predicts worsening glucose tolerance.[2]
References
- ^ Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E, RISC Study Group (2010). "alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population". PLOS ONE. 5 (5): 10883. Bibcode:2010PLoSO...510883G. doi:10.1371/journal.pone.0010883. PMC 2878333. PMID 20526369.
- ^ Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE (2013). "Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance". Diabetes. 62 (5): 1730–1737. doi:10.2337/db12-0707. PMC 3636608. PMID 23160532.













