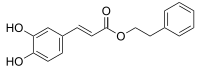
Caffeic acid phenethyl ester
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Phenylethyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | |
| Other names Phenylethyl caffeate; Phenethyl caffeate; Caffeic acid 2-phenylethyl ester; β-Phenylethyl caffeate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | CAPE |
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.155.538 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C17H16O4 |
| Molar mass | 284.311 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Caffeic acid phenethyl ester (CAPE) is a natural phenolic chemical compound. It is the ester of caffeic acid and phenethyl alcohol.
Natural occurrences
CAPE is found in a variety of plants. It is also a component of propolis from honeybee hives.[1]
Potential pharmacology
A variety of in vitro pharmacology and effects in animal models have been reported for CAPE, but their clinical significance is unknown. It has antimitogenic, anticarcinogenic, anti-inflammatory, and immunomodulatory properties in vitro.[2] Another study also showed that CAPE suppresses acute immune and inflammatory responses in vitro.[3] This anti-cancer effect was also seen when mice skin was treated with bee propolis and exposed to TPA, a chemical that induced skin papillomas. CAPE significantly reduced the number of papillomas.[4][5]
Recent study suggest that CAPE can also help in reducing oxidative stress caused by traumatic brain injury.[6]
References
- ^ Demestre M, Messerli SM, Celli N, et al. (August 2008). "CAPE (caffeic acid phenethyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice". Phytother Res. 23 (2): 226–30. doi:10.1002/ptr.2594. PMID 18726924. S2CID 21934712.
- ^ Natarajan K, Singh S, Burke TR, Grunberger D, Aggarwal BB (August 1996). "Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B". Proc. Natl. Acad. Sci. U.S.A. 93 (17): 9090–5. Bibcode:1996PNAS...93.9090N. doi:10.1073/pnas.93.17.9090. PMC 38600. PMID 8799159.
- ^ Orban Z, Mitsiades N, Burke TR, Tsokos M, Chrousos GP (2000). "Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation". Neuroimmunomodulation. 7 (2): 99–105. doi:10.1159/000026427. PMID 10686520. S2CID 31950905.
- ^ Huang MT, Ma W, Yen P, et al. (April 1996). "Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells". Carcinogenesis. 17 (4): 761–5. doi:10.1093/carcin/17.4.761. PMID 8625488.
- ^ Huang MT, Smart RC, Wong CQ, Conney AH (November 1988). "Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate". Cancer Res. 48 (21): 5941–6. PMID 3139287.
- ^ Nasution, Rizha Anshori; Asadul Islam, Andi; Hatta, Mochammad; (none), Prihantono; Turchan, Agus; (none), Nasrullah; Faruk, Muhammad (September 2020). "Role of CAPE in reducing oxidative stress in animal models with traumatic brain injury". Annals of Medicine and Surgery. 57: 118–122. doi:10.1016/j.amsu.2020.07.036. PMC 7390826. PMID 32760580.
- v
- t
- e
| Precursor | |
|---|---|
| Monohydroxycinnamic acids (Coumaric acids) |
|
| Dihydroxycinnamic acids |
|
| Trihydroxycinnamic acids |
|
| O-methylated forms | |
| others |
| glycoside-likes |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tartaric acid esters |
| ||||||||
| Other esters with caffeic acid |
| ||||||||
| Caffeoyl phenylethanoid glycoside (CPG) |
|
| Dimers |
|
|---|---|
| Trimers |
|
| Tetramers |
|
coenzyme A (CoA)
- Caffeoyl-CoA
- Cinnamoyl-CoA
- Coumaroyl-CoA










