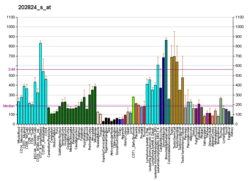
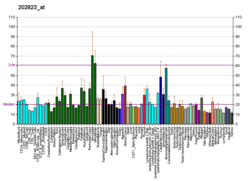
ELOC
| ELOC | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ELOC, SIII, eloC, TCEB1, transcription elongation factor B subunit 1, elongin C | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 600788; MGI: 1915173; HomoloGene: 38083; GeneCards: ELOC; OMA:ELOC - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Elongin C is a protein that in humans is encoded by the ELOC gene.[5][6]
Function
Elongin C is a subunit of the transcription factor B (SIII) complex. The SIII complex is composed of elongins A/A2, B and C. It activates elongation by RNA polymerase II by suppressing transient pausing of the polymerase at many sites within transcription units. Elongin A functions as the transcriptionally active component of the SIII complex, whereas elongins B and C are regulatory subunits. Elongin A2 is specifically expressed in the testis, and capable of forming a stable complex with elongins B and C. The von Hippel-Lindau tumor suppressor protein binds to elongins B and C, and thereby inhibits transcription elongation.[7]
Interactions
TCEB1 has been shown to interact with:
- TCEB2[8][9][10][11] and
- Von Hippel-Lindau tumor suppressor.[9][11][12][13][14][15][16]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000154582 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000079658 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Garrett KP, Haque D, Conaway RC, Conaway JW (Feb 1995). "A human cDNA encoding the small subunit of RNA polymerase II transcription factor SIII". Gene. 150 (2): 413–4. doi:10.1016/0378-1119(94)90467-7. PMID 7821821.
- ^ Duan DR, Pause A, Burgess WH, Aso T, Chen DY, Garrett KP, Conaway RC, Conaway JW, Linehan WM, Klausner RD (Oct 1995). "Inhibition of transcription elongation by the VHL tumor suppressor protein". Science. 269 (5229): 1402–6. Bibcode:1995Sci...269.1402D. doi:10.1126/science.7660122. PMID 7660122. S2CID 22334719.
- ^ "Entrez Gene: TCEB1 transcription elongation factor B (SIII), polypeptide 1 (15kDa, elongin C)".
- ^ Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (Oct 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- ^ a b Ohh M, Takagi Y, Aso T, Stebbins CE, Pavletich NP, Zbar B, Conaway RC, Conaway JW, Kaelin WG (Dec 1999). "Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein". J. Clin. Invest. 104 (11): 1583–91. doi:10.1172/JCI8161. PMC 481054. PMID 10587522.
- ^ Krumm A, Groudine M (Sep 1995). "Tumor suppression and transcription elongation: the dire consequences of changing partners". Science. 269 (5229): 1400–1. Bibcode:1995Sci...269.1400K. doi:10.1126/science.7660121. PMID 7660121. S2CID 39758696.
- ^ a b Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G (Feb 2002). "Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein". J. Biol. Chem. 277 (7): 4656–62. doi:10.1074/jbc.M108269200. PMID 11739384.
- ^ Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3: 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- ^ Tsuchiya H, Iseda T, Hino O (Jul 1996). "Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product". Cancer Res. 56 (13): 2881–5. PMID 8674032.
- ^ Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Pavletich NP (Jun 2002). "Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling". Science. 296 (5574): 1886–9. Bibcode:2002Sci...296.1886M. doi:10.1126/science.1073440. PMID 12004076. S2CID 19641938.
- ^ Hacker KE, Lee CM, Rathmell WK (2008). Zhang B (ed.). "VHL type 2B mutations retain VBC complex form and function". PLOS ONE. 3 (11): e3801. Bibcode:2008PLoSO...3.3801H. doi:10.1371/journal.pone.0003801. PMC 2583047. PMID 19030229.
- ^ Kim BY, Kim H, Cho EJ, Youn HD (Feb 2008). "Nur77 upregulates HIF-alpha by inhibiting pVHL-mediated degradation". Exp. Mol. Med. 40 (1): 71–83. doi:10.3858/emm.2008.40.1.71. PMC 2679322. PMID 18305400.
Further reading
- Aso T, Lane WS, Conaway JW, Conaway RC (1995). "Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II". Science. 269 (5229): 1439–43. Bibcode:1995Sci...269.1439A. doi:10.1126/science.7660129. PMID 7660129. S2CID 37649908.
- Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG (1995). "Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C". Science. 269 (5229): 1444–6. Bibcode:1995Sci...269.1444K. doi:10.1126/science.7660130. PMID 7660130. S2CID 25410108.
- Bradsher JN, Jackson KW, Conaway RC, Conaway JW (1994). "RNA polymerase II transcription factor SIII. I. Identification, purification, and properties". J. Biol. Chem. 268 (34): 25587–93. doi:10.1016/S0021-9258(19)74431-7. PMID 8244996.
- Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM, Klausner RD (1997). "The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins". Proc. Natl. Acad. Sci. U.S.A. 94 (6): 2156–61. Bibcode:1997PNAS...94.2156P. doi:10.1073/pnas.94.6.2156. PMC 20057. PMID 9122164.
- Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW, Kaelin WG (1998). "Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2". Mol. Cell. Biol. 18 (2): 732–41. doi:10.1128/mcb.18.2.732. PMC 108784. PMID 9447969.
- Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, Kile BJ, Kent SB, Alexander WS, Metcalf D, Hilton DJ, Nicola NA, Baca M (1999). "The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation". Proc. Natl. Acad. Sci. U.S.A. 96 (5): 2071–6. Bibcode:1999PNAS...96.2071Z. doi:10.1073/pnas.96.5.2071. PMC 26738. PMID 10051596.
- Stebbins CE, Kaelin WG, Pavletich NP (1999). "Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function". Science. 284 (5413): 455–61. Bibcode:1999Sci...284..455S. doi:10.1126/science.284.5413.455. PMID 10205047.
- Pause A, Peterson B, Schaffar G, Stearman R, Klausner RD (1999). "Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein". Proc. Natl. Acad. Sci. U.S.A. 96 (17): 9533–8. Bibcode:1999PNAS...96.9533P. doi:10.1073/pnas.96.17.9533. PMC 22243. PMID 10449727.
- Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, Klausner RD, Pause A (1999). "Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex". Proc. Natl. Acad. Sci. U.S.A. 96 (22): 12436–41. Bibcode:1999PNAS...9612436I. doi:10.1073/pnas.96.22.12436. PMC 22941. PMID 10535940.
- Ohh M, Takagi Y, Aso T, Stebbins CE, Pavletich NP, Zbar B, Conaway RC, Conaway JW, Kaelin WG (2000). "Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel-Lindau protein". J. Clin. Invest. 104 (11): 1583–91. doi:10.1172/JCI8161. PMC 481054. PMID 10587522.
- Aso T, Yamazaki K, Amimoto K, Kuroiwa A, Higashi H, Matsuda Y, Kitajima S, Hatakeyama M (2000). "Identification and characterization of Elongin A2, a new member of the Elongin family of transcription elongation factors, specifically expressed in the testis". J. Biol. Chem. 275 (9): 6546–52. doi:10.1074/jbc.275.9.6546. PMID 10692460.
- Adryan B, Decker HJ, Papas TS, Hsu T (2000). "Tracheal development and the von Hippel-Lindau tumor suppressor homolog in Drosophila". Oncogene. 19 (24): 2803–11. doi:10.1038/sj.onc.1203611. PMID 10851083.
- Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW (2000). "Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex". Proc. Natl. Acad. Sci. U.S.A. 97 (19): 10430–5. Bibcode:2000PNAS...9710430K. doi:10.1073/pnas.190332597. PMC 27041. PMID 10973499.
- Aso T, Yamazaki K, Aigaki T, Kitajima S (2000). "Drosophila von Hippel-Lindau tumor suppressor complex possesses E3 ubiquitin ligase activity". Biochem. Biophys. Res. Commun. 276 (1): 355–61. doi:10.1006/bbrc.2000.3451. PMID 11006129.
- Kamura T, Burian D, Yan Q, Schmidt SL, Lane WS, Querido E, Branton PE, Shilatifard A, Conaway RC, Conaway JW (2001). "Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase". J. Biol. Chem. 276 (32): 29748–53. doi:10.1074/jbc.M103093200. PMID 11384984.
- Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G (2002). "Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein". J. Biol. Chem. 277 (7): 4656–62. doi:10.1074/jbc.M108269200. PMID 11739384.
- Yamazaki K, Guo L, Sugahara K, Zhang C, Enzan H, Nakabeppu Y, Kitajima S, Aso T (2002). "Identification and biochemical characterization of a novel transcription elongation factor, Elongin A3". J. Biol. Chem. 277 (29): 26444–51. doi:10.1074/jbc.M202859200. PMID 11994304.
- Porkka K, Saramäki O, Tanner M, Visakorpi T (2002). "Amplification and overexpression of Elongin C gene discovered in prostate cancer by cDNA microarrays". Lab. Invest. 82 (5): 629–37. doi:10.1038/labinvest.3780457. PMID 12004003.
- v
- t
- e
-
 1lm8: Structure of a HIF-1a-pVHL-ElonginB-ElonginC Complex
1lm8: Structure of a HIF-1a-pVHL-ElonginB-ElonginC Complex -
 1lqb: Crystal structure of a hydroxylated HIF-1 alpha peptide bound to the pVHL/elongin-C/elongin-B complex
1lqb: Crystal structure of a hydroxylated HIF-1 alpha peptide bound to the pVHL/elongin-C/elongin-B complex -
 1vcb: THE VHL-ELONGINC-ELONGINB STRUCTURE
1vcb: THE VHL-ELONGINC-ELONGINB STRUCTURE -
 2c9w: CRYSTAL STRUCTURE OF SOCS-2 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 1.9A RESOLUTION
2c9w: CRYSTAL STRUCTURE OF SOCS-2 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 1.9A RESOLUTION -
 2fnj: Crystal structure of a B30.2/SPRY domain-containing protein GUSTAVUS in complex with Elongin B and Elongin C
2fnj: Crystal structure of a B30.2/SPRY domain-containing protein GUSTAVUS in complex with Elongin B and Elongin C -
 2izv: CRYSTAL STRUCTURE OF SOCS-4 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 2.55A RESOLUTION
2izv: CRYSTAL STRUCTURE OF SOCS-4 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 2.55A RESOLUTION