Fanetizole
Chemical compound
 | |
| Pharmacokinetic data | |
|---|---|
| Protein binding | % |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C17H16N2S |
| Molar mass | 280.39 g·mol−1 |
| 3D model (JSmol) |
|
| |
InChI
| |
| (verify) | |
Fanetizole is a drug that has immunoregulating activity.[citation needed]
Synthesis
Reaction of β-phenethylamine with ammonium thiocyanate gives the thiourea (2). Treatment of that product with phenacyl bromide produces fanetizole (3).[1]
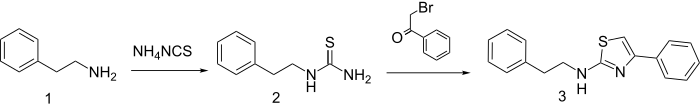
Fanetizole synthesis
References
- ^ Lombardino, J. G.; 1981, U.S. patent 4,307,106
- v
- t
- e
Histamine receptor modulators
| Agonists | |
|---|---|
| Antagonists |
|
| Agonists | |
|---|---|
| Antagonists |
|
| Agonists |
|
|---|---|
| Antagonists |
|
- See also
- Receptor/signaling modulators
- Monoamine metabolism modulators
- Monoamine reuptake inhibitors











