Orchinol
 | |
| Names | |
|---|---|
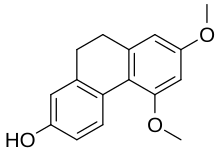
| Preferred IUPAC name 5,7-Dimethoxy-9,10-dihydrophenanthren-2-ol | |
| Other names 9,10-Dihydro-5,7-dimethoxyphenanthren-2-ol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C16H16O3 |
| Molar mass | 256.301 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Orchinol is a 9,10-dihydrophenanthrene, a type of phenanthrenoid. It can be isolated from infected Orchis militaris and infected Loroglossum hircinum[1] with Rhizoctonia repens.[2] This molecule has a phytoalexin effect. It reduces the growth of Cattleya aurantiaca seedlings[3] and has an antifungal activity.[4]
References
- ^ Structure of Orchinol, Loroglossol, and Hircinol. Roy M. Letcher and Llewellyn R. M. Nhamo, J. Chem. Soc., Perkin Trans. 1, 1973, pages 1263-1265, doi:10.1039/P19730001263
- ^ Orchinol. Richard Braun, Moderne Methoden der Pflanzenanalyse, 1963, Volume 6, pages 130-134, doi:10.1007/978-3-642-94878-7_7 (article in German)
- ^ Effects of Orchinol, Loroglossol, Dehydroorchinol, Batatasin III, and 3,4'- Dihydroxy-5-Methoxydihydrostilbene on Orchid Seedlings. Katherine A. Hills, Albert Stoessl, Allison P. Oliva and Joseph Arditti, Botanical Gazette, September 1984, Vol. 145, No. 3, pages 298-301 (link)
- ^ Structure and antifungal activity of hircinol, loroglossol and orchinol. M.H. Fisch, Brigitta H. Flick and J. Arditti, Phytochemistry, February 1973, Volume 12, Issue 2, Pages 437–441, doi:10.1016/0031-9422(73)80036-6
External links
- Orchinol at kanaya.naist.jp/knapsack_jsp
- Orchinol at WikiGenes
- v
- t
- e
Phenanthrenoids and their glycosides (molecules with a C6-C2-C6 backbone)
- 1-Phenanthrol
- 2-Phenanthrol
- 3-Phenanthrol
- 4-Phenanthrol
- 9-Phenanthrol
- coeloginanthrin
- confusaridin
- confusarin
- dehydroeffusol
- dehydrojuncusol
- flavanthrinin
- gymnopusin
- Nudol
- Perakensol
- plicatol A
- Plicatol B
- 2,3,4-trimethoxy-7,8-methylenedioxyphenanthrene
- 2,5-dihydroxy-3,4-dimethoxyphenanthrene
- 2,5-dihydroxy-3,4,9-trimethoxyphenanthrene
- 2,7-dihydroxy-3,4,6-trimethoxyphenanthrene
- 2,7-dihydroxy-3,4,9-trimethoxyphenanthrene
- 2,7-Dihydroxy-3,6-dimethoxyphenanthrene
- 3-hydroxy-2,4,-dimethoxy-7,8-methylenedioxyphenanthrene
- 2-hydroxy-3,5,7-trimethoxyphenanthrene
- 2-hydroxy-3,5,7-trimethoxy-9,10-dihydrophenanthrene
- 3,4,8-Trimethoxyphenanthrene-2,5-diol
- 4,6-dimethoxyphenanthrene-2,3,7-triol
- 4,9-dimethoxyphenanthrene-2,5-diol
- 7-Hydroxy-2,3,4,8-tetramethoxyphenanthrene
- coelogin
- coeloginin
- coeloginanthridin
- coelonin
- effusol
- hircinol
- juncusol
- loroglossol
- orchinol
- Plicatol C
- 4-methoxy-9,10-dihydrophenanthrene-2,3,7-triol
- 4,6-dimethoxy-9,10-dihydrophenanthrene-2,3,7-triol
- 9,10-dihydro-2,7-dihydroxy-3,4-dimethoxyphenanthrene
- 9,10-dihydro-2,7-dihydroxy-3,4,6-trimethoxyphenanthrene
- 9,10-Dihydro-2,5-dimethoxyphenanthrene-1,7-diol
- 9,10-dihydro-2,5-dihydroxy-3,4-dimethoxyphenanthrene
| Phenanthrene glycosides |
|
|---|---|
| 9,10-dihydrophenanthrene glycoside |
|
- cirrhopetalanthrin
- flavanthrin
- reptanthrin
- isoreptanthrin
- 8,8'-bidehydrojuncusol
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










