Sulfur tetrafluoride
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sulfur(IV) fluoride | |||
| Other names Sulfur tetrafluoride | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.029.103 | ||
PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 2418 | ||
CompTox Dashboard (EPA) |
| ||
InChI
| |||
| |||
| Properties | |||
Chemical formula | SF4 | ||
| Molar mass | 108.07 g/mol | ||
| Appearance | colorless gas | ||
| Density | 1.95 g/cm3, −78 °C | ||
| Melting point | −121.0 °C | ||
| Boiling point | −38 °C | ||
Solubility in water | reacts | ||
| Vapor pressure | 10.5 atm (22 °C)[1] | ||
| Structure | |||
| Seesaw (C2v) | |||
| 0.632 D[2] | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | highly reactive and toxic gas | ||
| NFPA 704 (fire diamond) |  3 0 2 W | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | none[1] | ||
REL (Recommended) | C 0.1 ppm (0.4 mg/m3)[1] | ||
IDLH (Immediate danger) | N.D.[1] | ||
| Safety data sheet (SDS) | ICSC 1456 | ||
| Related compounds | |||
Other anions | Sulfur dichloride Disulfur dibromide Sulfur trifluoride | ||
Other cations | Oxygen difluoride Selenium tetrafluoride Tellurium tetrafluoride | ||
Related sulfur fluorides | Disulfur difluoride Sulfur difluoride Disulfur decafluoride Sulfur hexafluoride | ||
Related compounds | Thionyl fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |||
Sulfur tetrafluoride is a chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluride is a useful reagent for the preparation of organofluorine compounds,[3] some of which are important in the pharmaceutical and specialty chemical industries.
Structure
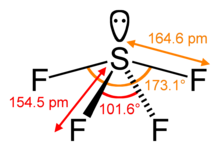
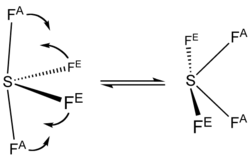
Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are S–Fax = 164.3 pm and S–Feq = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly.
The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation.[4]
Synthesis and manufacture
At the laboratory scale, sulfur tetrafluoride is prepared from elemental sulfur and cobaltic fluoride[5]
- S + 4CoF3 → SF4 + 4CoF2
SF4 is industrially produced by the reaction of SCl2 and NaF with acetonitrile as a catalyst[6]
- 3 SCl2 + 4 NaF → SF4 + S2Cl2 + 4 NaCl
At higher temperatures (e.g. 225–450 °C), the solvent is superfluous. Moreover, sulfur dichloride may be replaced by elemental sulfur (S) and chlorine (Cl2).[7][8]
A low-temperature (e.g. 20–86 °C) alternative to the chlorinative process above uses liquid bromine (Br2) as oxidant and solvent:[9]
- S(s) + 2 Br2(l; excess) + 4KF(s) → SF4↑ + 4 KBr(brom)
Use in synthesis of organofluorine compounds
In organic synthesis, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively.[10] The efficiency of these conversions are highly variable.
In the laboratory, the use of SF4 has been superseded by the safer and more easily handled diethylaminosulfur trifluoride, (C2H5)2NSF3, "DAST":[11] This reagent is prepared from SF4:[12]
- SF4 + (CH3)3SiN(C2H5)2 → (C2H5)2NSF3 + (CH3)3SiF
Other reactions
Sulfur chloride pentafluoride (SF
5Cl), a useful source of the SF5 group, is prepared from SF4.[13]
- SF4 + Cl2 + CsF → SF5Cl + CsCl
Hydrolysis of SF4 gives sulfur dioxide:[14]
- SF4 + 2 H2O → SO2 + 4 HF
This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF4 as a reagent.[6]
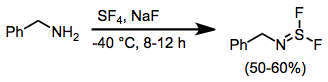
When amines are treated with SF4 and a base, aminosulfur difluorides result.[15]
Toxicity
SF
4 reacts inside the lungs with moisture, forming sulfur dioxide and hydrogen fluoride which forms highly toxic and corrosive hydrofluoric acid [16]
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0580". National Institute for Occupational Safety and Health (NIOSH).
- ^ Tolles, W. M.; W. M. Gwinn, W. D. (1962). "Structure and Dipole Moment for SF4". J. Chem. Phys. 36 (5): 1119–1121. Bibcode:1962JChPh..36.1119T. doi:10.1063/1.1732702.
- ^ Wang, C.-L. J. (2004). "Sulfur Tetrafluoride". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X. hdl:10261/236866. ISBN 9780471936237.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Kwasnik, W. (1963). "Fluorine compounds: Sulfur tetrafluoride". In Brauer, Georg (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1. Translated by Riley, Reed F. (2nd ed.). NY, NY: Academic Press. p. 168. LCCN 63-14307 – via the Internet Archive.
- ^ a b Fawcett, F. S.; Tullock, C. W. (1963). "Sulfur(IV) Fluoride" (PDF). Inorganic Syntheses. Vol. 7. pp. 119–124. doi:10.1002/9780470132388.ch33. ISBN 978-0-470-13166-4.
- ^ Tullock, C. W.; Fawcett, F. S.; Smith, W. C.; Coffman, D. D. (1960). "The Chemistry of Sulfur Tetrafluoride. I. The Synthesis of Sulfur Tetrafluoride". J. Am. Chem. Soc. 82 (3): 539–542. doi:10.1021/ja01488a011.
- ^ US 2992073, Tullock, C.W., "Synthesis of Sulfur Tetrafluoride", issued 1961
- ^ Winter, R.W.; Cook P.W. (2010). "A simplified and efficient bromine-facilitated SF4-preparation method". J. Fluorine Chem. 131: 780-783. doi:10.1016/j.jfluchem.2010.03.016
- ^ Hasek, W. R. (1961). "1,1,1-Trifluoroheptane". Organic Syntheses. 41: 104. doi:10.15227/orgsyn.041.0104.
- ^ Fauq, A. H. (2004). "N,N-Diethylaminosulfur Trifluoride". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X. hdl:10261/236866. ISBN 9780471936237..
- ^ W. J. Middleton; E. M. Bingham (1977). "Diethylaminosulfur Trifluoride". Organic Syntheses. 57: 440. doi:10.15227/orgsyn.057.0050.
- ^ Nyman, F.; Roberts, H. L.; Seaton, T. (1966). "Sulfur Chloride Pentafluoride" (PDF). Inorganic Syntheses. Vol. 8. McGraw-Hill. p. 160. doi:10.1002/9780470132395.ch42. ISBN 9780470132395.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Grunwell, John R.; Dye, Sandra L. (1975). "Novel generation of benzonitrile-N-sulfide". Tetrahedron Letters. 16 (21): 1739–1740. doi:10.1016/s0040-4039(00)72247-7. ISSN 0040-4039.
- ^ Johnston, H. (2003). A Bridge not Attacked: Chemical Warfare Civilian Research During World War II. World Scientific. pp. 33–36. ISBN 981-238-153-8.












