Dehidrataza delta aminolevulinske kiseline je enzim koji je kod ljudi kodiran ALAD genom.[4][5]
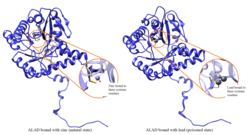
ALAD enzim se sastoji od 8 identičnih podjedinica i katalizuje kondenzaciju 2 molekula delta-aminolevulinata, čime se formira porfobilinogen (prekurzor hema, citohroma i drugih hemoproteina). ALAD katalizuje drugi korak u porfirinskom i hemnom biosintetičkom putu. Cink je esencijalan za enzimatsku aktivnost. Olovo inhibira ALAD, počevši od krvnih nivoa olova koji su nekad smatrani bezbednim (<10 μg/dL) i postoji negativna korelacija duž opseg od 5 do 95 μg/dL.[6] Inhibicija ALAD-a olovom dovodi do anemije, prvenstveno zbog inhibicije sinteze hema i skraćivanja životnog veka cirkulišućih crvenih krvnih zrnaca, ali i zbog stimulisanja prekomerne produkcije hormona eritropoietina, što dovodi do neadekvatne maturacije crvenih krvnih zrnaca iz njihovih progenitora. Defekat u ALAD strukturnom genu može da dovede do povećane senzitivnosti na trovanje olovom i akutne hepatičke porfirije. Alternativno splajsovane transkriptne varijante koje kodiraju različite izoforme su identifikovane.[7]
Vidi još
Reference
- ^ а б в GRCm38: Ensembl release 89: ENSMUSG00000028393 - Ensembl, May 2017
- ^ „Human PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ „Mouse PubMed Reference:”. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Eiberg H, Mohr J, Nielsen LS (1983). „delta-Aminolevulinatedehydrase: synteny with ABO-AK1-ORM (and assignment to chromosome 9)”. Clin Genet. 23 (2): 150—4. PMID 6839527. doi:10.1111/j.1399-0004.1983.tb01864.x.
- ^ Beaumont C; Foubert C; Grandchamp B; Weil D; Van Cong N'Guyen; Gross MS; Nordmann Y (1984). „Assignment of the human gene for delta aminolevulinate dehydrase to chromosome 9 by somatic cell hybridization and specific enzyme immunoassay”. Ann Hum Genet. 48 (Pt 2): 153—9. PMID 6378062. doi:10.1111/j.1469-1809.1984.tb01010.x.
- ^ Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, Citra M, Quinones A, Bosch SJ, Swarts SG (2007). Toxicological Profile for Lead (PDF). Atlanta, GA: Agency for Toxic Substances and Disease Registry (US). стр. 22, 30. PMID 24049859. Приступљено 22. 11. 2015.
- ^ „Entrez Gene: ALAD aminolevulinate, delta-, dehydratase”.
Literatura
- Bernard A, Lauwerys R (1988). „Metal-induced alterations of delta-aminolevulinic acid dehydratase.”. Ann. N. Y. Acad. Sci. 514: 41—7. PMID 3327436. doi:10.1111/j.1749-6632.1987.tb48759.x.
- Jaffe EK (2005). „The porphobilinogen synthase catalyzed reaction mechanism.”. Bioorg. Chem. 32 (5): 316—25. PMID 15381398. doi:10.1016/j.bioorg.2004.05.010.
- Roels HA, Buchet JP, Lauwerys RR, Sonnet J (1975). „Comparison of in vivo effect of inorganic lead and cadmium on glutathione reductase system and delta-aminolevulinate dehydratase in human erythrocytes.”. British journal of industrial medicine. 32 (3): 181—92. PMC 1008057
 . PMID 1156566. doi:10.1136/oem.32.3.181.
. PMID 1156566. doi:10.1136/oem.32.3.181. - Ishida N, Fujita H, Fukuda Y, et al. (1992). „Cloning and expression of the defective genes from a patient with delta-aminolevulinate dehydratase porphyria.”. J. Clin. Invest. 89 (5): 1431—7. PMC 443012
 . PMID 1569184. doi:10.1172/JCI115732.
. PMID 1569184. doi:10.1172/JCI115732. - Dawson SJ, White LA (1992). „Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin.”. J. Infect. 24 (3): 317—20. PMID 1602151. doi:10.1016/S0163-4453(05)80037-4.
- Astrin KH, Kaya AH, Wetmur JG, Desnick RJ (1991). „RsaI polymorphism in the human delta-aminolevulinate dehydratase gene at 9q34.”. Nucleic Acids Res. 19 (15): 4307. PMC 328595
 . PMID 1678509. doi:10.1093/nar/19.15.4307-a.
. PMID 1678509. doi:10.1093/nar/19.15.4307-a. - Wetmur JG, Kaya AH, Plewinska M, Desnick RJ (1991). „Molecular characterization of the human delta-aminolevulinate dehydratase 2 (ALAD2) allele: implications for molecular screening of individuals for genetic susceptibility to lead poisoning.”. Am. J. Hum. Genet. 49 (4): 757—63. PMC 1683158
 . PMID 1716854.
. PMID 1716854. - Plewinska M, Thunell S, Holmberg L, et al. (1991). „delta-Aminolevulinate dehydratase deficient porphyria: identification of the molecular lesions in a severely affected homozygote.”. Am. J. Hum. Genet. 49 (1): 167—74. PMC 1683193
 . PMID 2063868.
. PMID 2063868. - Potluri VR, Astrin KH, Wetmur JG, et al. (1987). „Human delta-aminolevulinate dehydratase: chromosomal localization to 9q34 by in situ hybridization.”. Hum. Genet. 76 (3): 236—9. PMID 3036687. doi:10.1007/BF00283614.
- Gibbs PN, Jordan PM (1986). „Identification of lysine at the active site of human 5-aminolaevulinate dehydratase.”. Biochem. J. 236 (2): 447—51. PMC 1146860
 . PMID 3092810.
. PMID 3092810. - Wetmur JG, Bishop DF, Cantelmo C, Desnick RJ (1986). „Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone.”. Proc. Natl. Acad. Sci. U.S.A. 83 (20): 7703—7. PMC 386789
 . PMID 3463993. doi:10.1073/pnas.83.20.7703.
. PMID 3463993. doi:10.1073/pnas.83.20.7703. - Wetmur JG, Bishop DF, Ostasiewicz L, Desnick RJ (1986). „Molecular cloning of a cDNA for human delta-aminolevulinate dehydratase.”. Gene. 43 (1-2): 123—30. PMID 3758678. doi:10.1016/0378-1119(86)90015-6.
- Doss M, von Tiepermann R, Schneider J (1981). „Acute hepatic porphyria syndrome with porphobilinogen synthase defect.”. Int. J. Biochem. 12 (5-6): 823—6. PMID 7450139. doi:10.1016/0020-711X(80)90170-6.
- Kaya AH, Plewinska M, Wong DM, et al. (1994). „Human delta-aminolevulinate dehydratase (ALAD) gene: structure and alternative splicing of the erythroid and housekeeping mRNAs.”. Genomics. 19 (2): 242—8. PMID 8188255. doi:10.1006/geno.1994.1054.
- Akagi R, Yasui Y, Harper P, Sassa S (1999). „A novel mutation of delta-aminolaevulinate dehydratase in a healthy child with 12% erythrocyte enzyme activity.”. Br. J. Haematol. 106 (4): 931—7. PMID 10519994. doi:10.1046/j.1365-2141.1999.01647.x.
- Akagi R, Shimizu R, Furuyama K, et al. (2000). „Novel molecular defects of the delta-aminolevulinate dehydratase gene in a patient with inherited acute hepatic porphyria.”. Hepatology. 31 (3): 704—8. PMID 10706561. doi:10.1002/hep.510310321.
- Kervinen J, Jaffe EK, Stauffer F, et al. (2001). „Mechanistic basis for suicide inactivation of porphobilinogen synthase by 4,7-dioxosebacic acid, an inhibitor that shows dramatic species selectivity.”. Biochemistry. 40 (28): 8227—36. PMID 11444968. doi:10.1021/bi010656k.
Нормативна контрола  | |
|---|

 . PMID 1156566. doi:10.1136/oem.32.3.181.
. PMID 1156566. doi:10.1136/oem.32.3.181.  . PMID 1569184. doi:10.1172/JCI115732.
. PMID 1569184. doi:10.1172/JCI115732.  . PMID 1678509. doi:10.1093/nar/19.15.4307-a.
. PMID 1678509. doi:10.1093/nar/19.15.4307-a.  . PMID 1716854.
. PMID 1716854.  . PMID 2063868.
. PMID 2063868.  . PMID 3092810.
. PMID 3092810.  . PMID 3463993. doi:10.1073/pnas.83.20.7703.
. PMID 3463993. doi:10.1073/pnas.83.20.7703. 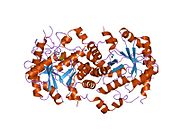 1e51: Kristalna struktura ljudske eritrocitne dehidrataze 5-aminolaevulinske kiseline
1e51: Kristalna struktura ljudske eritrocitne dehidrataze 5-aminolaevulinske kiseline 1pv8: Kristalna struktura F12L mutanta niskog afiniteta ljudske forfobilinogene sintaze
1pv8: Kristalna struktura F12L mutanta niskog afiniteta ljudske forfobilinogene sintaze

















