Tin(IV) fluoride
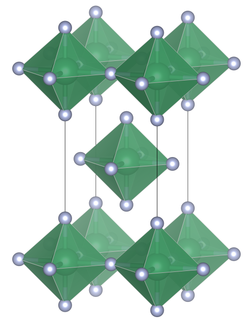 Unit cell of tin(IV) fluoride | |
| Names | |
|---|---|
| IUPAC name tin(IV) fluoride | |
| Other names stannic fluoride, tin tetrafluoride | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ECHA InfoCard | 100.029.105 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
InChI
| |
| |
| Properties | |
Chemical formula | SnF4 |
| Molar mass | 194.704 g/mol |
| Appearance | white solid |
| Melting point | above 700 °C (sublimes) |
| Structure | |
Crystal structure | Tetragonal, tI10 |
Space group | I4/mmm, No. 139 |
| Related compounds | |
Other anions | Tin(IV) chloride Tin(IV) bromide Tin(IV) iodide |
Other cations | Carbon tetrafluoride Silicon tetrafluoride Germanium tetrafluoride Tin tetrafluoride Lead tetrafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4 and is a white solid with a melting point above 700 °C.[1]
SnF4 can be prepared by the reaction of tin metal with fluorine gas:[2]
- Sn + 2F2 → SnF4
However, a passivating metal fluoride layer will be created and the surface will eventually become unreactive. An alternative synthesis is the reaction of SnCl4 with anhydrous hydrogen fluoride:[1]
- SnCl4 + 4HF → SnF4 + 4HCl
With alkali metal fluorides (e.g. KF) hexafluorostannates are produced (e.g.K2SnF6), which contain the octahedral SnF62− anion. SnF4 behaves as a Lewis acid and adducts L2·SnF4 and L·SnF4 have been produced.[2]
Structure
Unlike the other tin tetrahalides, tin(IV) chloride, tin(IV) bromide, and tin(IV) iodide, which contain tetrahedrally coordinated tin, tin(IV) fluoride contains planar layers of octahedrally coordinated tin, where the octahedra share four corners and there are two terminal, unshared, fluorine atoms trans to one another.[3] The melting point of SnF4 is much higher (700 °C) than the other tin(IV) halides which are relatively low melting, (SnCl4, −33.3 °C; SnBr4, 31 °C; SnI4, 144 °C).[1] The structure can also be contrasted with the tetrafluorides of the lighter members of group 14, (CF4, SiF4 and GeF4) which in the solid state form molecular crystals.[2]
See also
References
- ^ a b c Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford:Butterworth-Heinemann. pp. 381. ISBN 0-7506-3365-4.
- ^ a b c Holleman, A. F.; Wiberg, E.; Wiberg, N. (2001). Inorganic Chemistry, 1st Edition. Academic Press. p. 908. ISBN 0-12-352651-5.
- ^ Inorganic Chemistry [Paperback],2d Edition, Housecroft, Sharpe,2004, Pearson Education ISBN 0130399132, ISBN 978-0130399137
- v
- t
- e
- SnBr4
- SnCl4
- SnF4
- SnH4
- SnI4
- SnO2
- SnS2
- Sn(CH3COO)4
- Sn(NO3)4
- Sn(IO3)4












