Protein-coding gene in the species Homo sapiens
| More reference expression data |
| BioGPS | 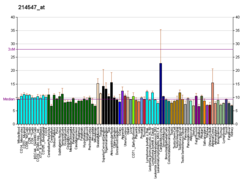
 | | More reference expression data |
|
|---|
| Gene ontology |
|---|
| Molecular function | - nucleotide binding
- manganese ion binding
- metal ion binding
- lyase activity
- adenylate cyclase activity
- phosphorus-oxygen lyase activity
- bicarbonate binding
- ATP binding
- magnesium ion binding
- ATPase binding
| | Cellular component | - cytoplasm
- cytosol
- cell projection
- membrane
- microtubule cytoskeleton
- growth cone
- basal part of cell
- plasma membrane
- apical part of cell
- cilium
- axon
- neuronal cell body
- dendrite
- mitochondrion
- perinuclear region of cytoplasm
- motile cilium
- cytoskeleton
- nucleus
- apical plasma membrane
- extracellular region
| | Biological process | - cellular response to inorganic substance
- intracellular signal transduction
- epithelial cilium movement involved in extracellular fluid movement
- cyclic nucleotide biosynthetic process
- spermatogenesis
- positive regulation of apoptotic process
- cAMP biosynthetic process
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001167749
NM_001297772
NM_018417 |
| |
|---|
| RefSeq (protein) | |
|---|
NP_001161221
NP_001284701
NP_060887 |
| |
|---|
| Location (UCSC) | Chr 1: 167.81 – 167.91 Mb | Chr 1: 165.31 – 165.4 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Adenylyl cyclase 10 also known as ADCY10 is an enzyme that, in humans, is encoded by the ADCY10 gene.[5]
Function
The protein encoded by this gene belongs to a distinct class of mammalian adenylyl cyclase that is soluble and insensitive to G protein or forskolin regulation. It is localized in the cytoplasm and is thought to function as a general bicarbonate sensor throughout the body. It may also play an important role in the generation of cAMP in spermatozoa, implying possible roles in sperm maturation through the epididymis, capacitation, hypermotility, and/or the acrosome reaction.[6]
Clinical significance
Mutations in the ADCY10 gene are associated with an increased risk of adsorptive hypercalciuria[5] and male infertility.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000143199 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000026567 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Reed BY, Heller HJ, Gitomer WL, Pak CY (November 1999). "Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24". The Journal of Clinical Endocrinology and Metabolism. 84 (11): 3907–3913. doi:10.1210/jcem.84.11.6155. PMID 10566627.
- ^ "Entrez Gene: ADCY10".
- ^ Akbari A, Pipitone GB, Anvar Z, Jaafarinia M, Ferrari M, Carrera P, et al. (June 2019). "ADCY10 frameshift variant leading to severe recessive asthenozoospermia and segregating with absorptive hypercalciuria". Human Reproduction. 34 (6): 1155–1164. doi:10.1093/humrep/dez048. PMID 31119281.
External links
Further reading
- Hukovic N, Panetta R, Kumar U, Rocheville M, Patel YC (August 1998). "The cytoplasmic tail of the human somatostatin receptor type 5 is crucial for interaction with adenylyl cyclase and in mediating desensitization and internalization". The Journal of Biological Chemistry. 273 (33): 21416–21422. doi:10.1074/jbc.273.33.21416. PMID 9694905.
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR (January 1999). "Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals". Proceedings of the National Academy of Sciences of the United States of America. 96 (1): 79–84. Bibcode:1999PNAS...96...79B. doi:10.1073/pnas.96.1.79. PMC 15096. PMID 9874775.
- Hayes JS, Lawler OA, Walsh MT, Kinsella BT (August 1999). "The prostacyclin receptor is isoprenylated. Isoprenylation is required for efficient receptor-effector coupling". The Journal of Biological Chemistry. 274 (34): 23707–23718. doi:10.1074/jbc.274.34.23707. PMID 10446129.
- Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, et al. (May 2000). "Specific expression of soluble adenylyl cyclase in male germ cells". Molecular Reproduction and Development. 56 (1): 6–11. doi:10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. PMID 10737962. S2CID 34539645.
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al. (July 2000). "Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor". Science. 289 (5479): 625–628. Bibcode:2000Sci...289..625C. doi:10.1126/science.289.5479.625. PMID 10915626.
- Jaiswal BS, Conti M (August 2001). "Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase". The Journal of Biological Chemistry. 276 (34): 31698–31708. doi:10.1074/jbc.M011698200. PMID 11423534.
- Reed BY, Gitomer WL, Heller HJ, Hsu MC, Lemke M, Padalino P, et al. (April 2002). "Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spinal bone density". The Journal of Clinical Endocrinology and Metabolism. 87 (4): 1476–1485. doi:10.1210/jc.87.4.1476. PMID 11932268.
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, et al. (January 2003). "Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains". FASEB Journal. 17 (1): 82–84. doi:10.1096/fj.02-0598fje. PMID 12475901. S2CID 22099561.
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR (May 2003). "Kinetic properties of "soluble" adenylyl cyclase. Synergism between calcium and bicarbonate". The Journal of Biological Chemistry. 278 (18): 15922–15926. doi:10.1074/jbc.M212475200. PMID 12609998.
- Jaiswal BS, Conti M (September 2003). "Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa". Proceedings of the National Academy of Sciences of the United States of America. 100 (19): 10676–10681. Bibcode:2003PNAS..10010676J. doi:10.1073/pnas.1831008100. PMC 196863. PMID 12958208.
- Marjanovic JA, Li Z, Stojanovic A, Du X (November 2005). "Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation". The Journal of Biological Chemistry. 280 (45): 37430–37438. doi:10.1074/jbc.M506518200. PMID 16144836.
- Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, et al. (July 2007). "Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP". The Journal of General Physiology. 130 (1): 99–109. doi:10.1085/jgp.200709784. PMC 2154360. PMID 17591988.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
|
|---|
| Activity | |
|---|
| Regulation | |
|---|
| Classification | |
|---|
| Kinetics | |
|---|
| Types | |
|---|
Portal: Biology
Biology
 | This enzyme-related article is a stub. You can help Wikipedia by expanding it. |















