Protein-coding gene in the species Homo sapiens
| PTPN2 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
|
|
| Identifiers |
|---|
| Aliases | PTPN2, PTN2, PTPT, TC-PTP, TCELLPTP, TCPTP, protein tyrosine phosphatase, non-receptor type 2, protein tyrosine phosphatase non-receptor type 2 |
|---|
| External IDs | OMIM: 176887; MGI: 97806; HomoloGene: 7497; GeneCards: PTPN2; OMA:PTPN2 - orthologs |
|---|
| Gene location (Human) |
|---|
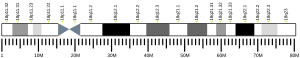
 | | Chr. | Chromosome 18 (human)[1] |
|---|
| | Band | 18p11.21 | Start | 12,785,478 bp[1] |
|---|
| End | 12,929,643 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 18 (mouse)[2] |
|---|
| | Band | 18|18 E1 | Start | 67,798,581 bp[2] |
|---|
| End | 67,857,665 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - tendon of biceps brachii
- monocyte
- granulocyte
- tonsil
- cartilage tissue
- parotid gland
- oocyte
- lymph node
- body of pancreas
- gonad
|
| | Top expressed in | - otic placode
- saccule
- condyle
- primary oocyte
- fossa
- Paneth cell
- hair follicle
- secondary oocyte
- submandibular gland
- primitive streak
|
| | More reference expression data |
|
|---|
| BioGPS | |
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein binding
- protein kinase binding
- phosphatase activity
- integrin binding
- phosphoprotein phosphatase activity
- syntaxin binding
- non-membrane spanning protein tyrosine phosphatase activity
- receptor tyrosine kinase binding
- hydrolase activity
- protein tyrosine phosphatase activity
- STAT family protein binding
| | Cellular component | - cytoplasm
- membrane
- plasma membrane
- endoplasmic reticulum
- nucleus
- endoplasmic reticulum-Golgi intermediate compartment
- nucleoplasm
- cytosol
| | Biological process | - negative regulation of type I interferon-mediated signaling pathway
- negative regulation of epidermal growth factor receptor signaling pathway
- positive regulation of PERK-mediated unfolded protein response
- dephosphorylation
- negative regulation of interleukin-4-mediated signaling pathway
- positive regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway
- negative regulation of interleukin-6-mediated signaling pathway
- negative regulation of cell population proliferation
- protein dephosphorylation
- negative regulation of interleukin-2-mediated signaling pathway
- regulation of interferon-gamma-mediated signaling pathway
- negative regulation of protein tyrosine kinase activity
- regulation of hepatocyte growth factor receptor signaling pathway
- negative regulation of platelet-derived growth factor receptor-beta signaling pathway
- insulin receptor signaling pathway
- negative regulation of interferon-gamma-mediated signaling pathway
- glucose homeostasis
- negative regulation of chemotaxis
- negative regulation of tumor necrosis factor-mediated signaling pathway
- negative regulation of macrophage differentiation
- negative regulation of T cell receptor signaling pathway
- negative regulation of positive thymic T cell selection
- negative regulation of insulin receptor signaling pathway
- T cell differentiation
- negative regulation of ERK1 and ERK2 cascade
- erythrocyte differentiation
- negative regulation of lipid storage
- B cell differentiation
- peptidyl-tyrosine dephosphorylation
- positive regulation of gluconeogenesis
- negative regulation of macrophage colony-stimulating factor signaling pathway
- negative regulation of inflammatory response
- negative regulation of transcription by RNA polymerase II
- negative regulation of tyrosine phosphorylation of STAT protein
- cellular response to cytokine stimulus
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001207013
NM_001308287
NM_002828
NM_080422
NM_080423 |
| |
|---|
| RefSeq (protein) | |
|---|
NP_001193942
NP_001295216
NP_002819
NP_536347
NP_536348 |
| |
|---|
| Location (UCSC) | Chr 18: 12.79 – 12.93 Mb | Chr 18: 67.8 – 67.86 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Tyrosine-protein phosphatase non-receptor type 2 is an enzyme that in humans is encoded by the PTPN2 gene.[5][6]
The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. Members of the PTP family share a highly conserved catalytic motif, which is essential for the catalytic activity. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation.
Epidermal growth factor receptor and the adaptor protein Shc were reported to be substrates of this PTP, which suggested the roles in growth factor mediated cell signaling. Three alternatively spliced variants of this gene, which encode isoforms differing at their extreme C-termini, have been described.
The different C-termini are thought to determine the substrate specificity, as well as the cellular localization of the isoforms. Two highly related but distinctly processed pseudogenes that localize to distinct chromosomes have been reported.[6]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000175354 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024539 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Brown-Shimer S, Johnson KA, Lawrence JB, Johnson C, Bruskin A, Green NR, Hill DE (Aug 1990). "Molecular cloning and chromosome mapping of the human gene encoding protein phosphotyrosyl phosphatase 1B". Proc Natl Acad Sci U S A. 87 (13): 5148–52. Bibcode:1990PNAS...87.5148B. doi:10.1073/pnas.87.13.5148. PMC 54279. PMID 2164224.
- ^ a b "Entrez Gene: PTPN2 protein tyrosine phosphatase, non-receptor type 2".
Further reading
- Baumgartner, CK, Ebrahimi-Nik, H, Iracheta-Vellve, A (2023). "The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity". Nature. 622 (7984): 850–862. Bibcode:2023Natur.622..850B. doi:10.1038/s41586-023-06575-7. PMC 10599993. PMID 37794185.
- LaFleur MW, Nguyen TH, Coxe MA (2019). "PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity". Nat. Immunol. 20 (10): 1335–1347. doi:10.1038/s41590-019-0480-4. PMC 6754306. PMID 31527834.
- Manguso RT, Pope HW, Zimmer MD (2017). "In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target". Nature. 547 (7664): 413–418. doi:10.1038/nature23270. PMC 5924693. PMID 28723893.
- Mosinger B, Tillmann U, Westphal H, Tremblay ML (1992). "Cloning and characterization of a mouse cDNA encoding a cytoplasmic protein-tyrosine-phosphatase". Proc. Natl. Acad. Sci. U.S.A. 89 (2): 499–503. Bibcode:1992PNAS...89..499M. doi:10.1073/pnas.89.2.499. PMC 48266. PMID 1731319.
- Swarup G, Kamatkar S, Radha V, Rema V (1991). "Molecular cloning and expression of a protein-tyrosine phosphatase showing homology with transcription factors Fos and Jun". FEBS Lett. 280 (1): 65–9. Bibcode:1991FEBSL.280...65S. doi:10.1016/0014-5793(91)80205-H. PMID 1849097. S2CID 10568838.
- Cool DE, Tonks NK, Charbonneau H, et al. (1989). "cDNA isolated from a human T-cell library encodes a member of the protein-tyrosine-phosphatase family". Proc. Natl. Acad. Sci. U.S.A. 86 (14): 5257–61. Bibcode:1989PNAS...86.5257C. doi:10.1073/pnas.86.14.5257. PMC 297600. PMID 2546150.
- Lorenzen JA, Dadabay CY, Fischer EH (1995). "COOH-terminal sequence motifs target the T cell protein tyrosine phosphatase to the ER and nucleus". J. Cell Biol. 131 (3): 631–43. doi:10.1083/jcb.131.3.631. PMC 2120615. PMID 7593185.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Johnson CV, Cool DE, Glaccum MB, et al. (1993). "Isolation and mapping of human T-cell protein tyrosine phosphatase sequences: localization of genes and pseudogenes discriminated using fluorescence hybridization with genomic versus cDNA probes". Genomics. 16 (3): 619–29. doi:10.1006/geno.1993.1239. PMID 8325634.
- Tiganis T, Flint AJ, Adam SA, Tonks NK (1997). "Association of the T-cell protein tyrosine phosphatase with nuclear import factor p97". J. Biol. Chem. 272 (34): 21548–57. doi:10.1074/jbc.272.34.21548. PMID 9261175.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Tiganis T, Bennett AM, Ravichandran KS, Tonks NK (1998). "Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase". Mol. Cell. Biol. 18 (3): 1622–34. doi:10.1128/MCB.18.3.1622. PMC 108877. PMID 9488479.
- Tiganis T, Kemp BE, Tonks NK (1999). "The protein-tyrosine phosphatase TCPTP regulates epidermal growth factor receptor-mediated and phosphatidylinositol 3-kinase-dependent signaling". J. Biol. Chem. 274 (39): 27768–75. doi:10.1074/jbc.274.39.27768. PMID 10488121.
- Iversen LF, Moller KB, Pedersen AK, et al. (2002). "Structure determination of T cell protein-tyrosine phosphatase". J. Biol. Chem. 277 (22): 19982–90. doi:10.1074/jbc.M200567200. PMID 11907034.
- Simoncic PD, Lee-Loy A, Barber DL, et al. (2002). "The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3". Curr. Biol. 12 (6): 446–53. Bibcode:2002CBio...12..446S. doi:10.1016/S0960-9822(02)00697-8. PMID 11909529. S2CID 16693906.
- ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, et al. (2002). "Identification of a nuclear Stat1 protein tyrosine phosphatase". Mol. Cell. Biol. 22 (16): 5662–8. doi:10.1128/MCB.22.16.5662-5668.2002. PMC 133976. PMID 12138178.
- Zhu W, Mustelin T, David M (2002). "Arginine methylation of STAT1 regulates its dephosphorylation by T cell protein tyrosine phosphatase". J. Biol. Chem. 277 (39): 35787–90. doi:10.1074/jbc.C200346200. PMID 12171910.
- Yamamoto T, Sekine Y, Kashima K, et al. (2002). "The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation". Biochem. Biophys. Res. Commun. 297 (4): 811–7. doi:10.1016/S0006-291X(02)02291-X. hdl:2115/28124. PMID 12359225.
- Gupta S, Radha V, Sudhakar Ch, Swarup G (2003). "A nuclear protein tyrosine phosphatase activates p53 and induces caspase-1-dependent apoptosis". FEBS Lett. 532 (1–2): 61–6. doi:10.1016/S0014-5793(02)03628-1. PMID 12459463. S2CID 33468275.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, et al. (2003). "Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP". Mol. Cell. Biol. 23 (6): 2096–108. doi:10.1128/MCB.23.6.2096-2108.2003. PMC 149470. PMID 12612081.
 | This protein-related article is a stub. You can help Wikipedia by expanding it. |

 1l8k: T Cell Protein-Tyrosine Phosphatase Structure
1l8k: T Cell Protein-Tyrosine Phosphatase Structure